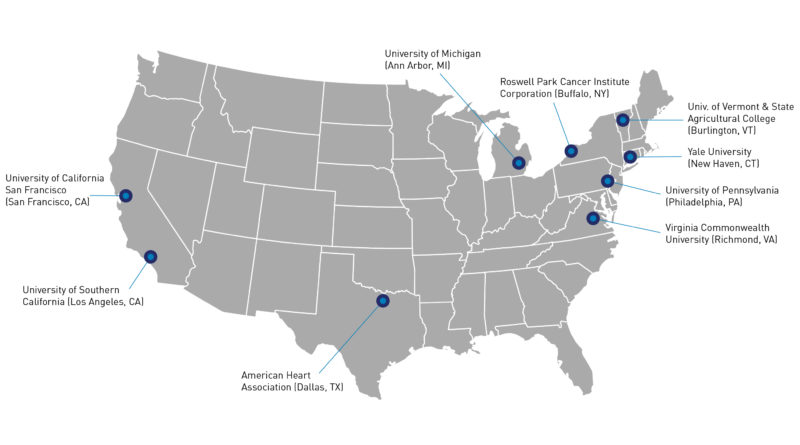
Tobacco Centers of Regulatory Science (TCORS)
FDA and NIH, as part of an on-going interagency partnership, awarded nine Tobacco Centers of Regulatory Science (TCORS) grants in September 2018 to support research to inform the regulation of tobacco products. In this second round of funding, the TCORS 2.0 research centers will receive, in total, more than $151 million in grants from FY 2018-2022.
What scientists learn about tobacco through the TCORS program helps inform and assess FDA’s prior, ongoing, and potential regulatory activities. TCORS investigators also have the flexibility and capacity to respond to FDA’s research needs as issues are raised in today’s rapidly evolving tobacco marketplace.
Tobacco Centers of Regulatory Science (TCORS)
Funded Research: Tobacco Regulatory Science Program
Investing in Tobacco Regulatory Science
The TCORS 2.0 program is designed to generate scientific evidence in seven scientific domains:
- Approaches that test the toxicity of tobacco smoke, aerosol, or specific constituents
- Effects of tobacco product characteristics on addiction and abuse liability
- Short and long term health effects of tobacco products
- Understanding of knowledge, attitudes and behaviors related to tobacco product use
- Understanding of how to effectively communicate the health effects of tobacco products
- Influences of tobacco marketing
- Understanding of the impact of potential FDA regulatory actions